March '25 Infectious Diseases Update
Posted by Doug Black, PharmD., Ann Lloyd, PharmD. on Mar 11th 2025
Sanford Guide ID Update features current developments in infectious diseases, curated by the Sanford Guide Editorial Board and our AMS Program Manager. Links marked with a * are available to Sanford Guide All Access & Sanford Guide for Web clients. All other links are available without a Sanford Guide subscription. To receive monthly updates via email, sign up now.
Article of the Month (Editors' Choice)
By Henry F. Chambers, MD
- Treatment of multidrug-resistant tuberculosis (MDR-TB), defined as resistance to both isoniazid and rifampin and always a challenge to treat, may have just become a bit easier with the publication of the much-anticipated results of the endTB clinical trial in the New England of Medicine (N Engl J Med 2025;392:468). TB is a global public health threat, with an estimated 10.6 million people developing TB in 2022 and 1.3 million deaths from the disease. About 410,000 new cases of multidrug-resistant or rifampicin-resistant TB are estimated to have occurred in 2022. There is a growing need for safe, more effective, better tolerated, and, ideally, shorter course treatment regimens.
- This phase 3 randomized controlled trial, begun in 2017, enrolled 754 participants (15 years of age and older) with fluoroquinolone-susceptible, rifampin-resistant pulmonary TB from seven countries (Georgia, India, Kazakhstan, Lesotho, Pakistan, Peru, and South Africa). Participants were randomized to one of five experimental nine-month oral regimens or to an 18-to-24-month standard therapy control arm. The 9-month oral regimens included various combinations of bedaquiline (B), delamanid (D), linezolid (L), levofloxacin (Lfx) or moxifloxacin (M), clofazimine (C), and pyrazinamide (Z). Three regimens, BLMZ, BCLLfxZ, and BDLLfxZ had favorable outcomes ranging from 85-90%, comparable to standard therapy with 81% favorable outcome.
- The three highly effective all-oral regimens identified in the endTB trial complement the highly effective six-month oral regimen of BPaLM (bedaquiline, pretomanid, linezolid, moxifloxacin), the only other proven effective, shorter-course regimen for treatment of MDR-TB. However, BPaLM is not suitable for all patients. The endTB regimens can be used to treat nearly all adults, children, adolescents, and pregnant women with fluoroquinolone-susceptible, pulmonary MDR-TB, including those with severe TB disease and HIV, regardless of CD4 count. All drugs in the endTB regimens have pediatric formulations and are recommended for treatment of TB regardless of age, and all are acceptable for use during pregnancy. This expansion in the number of effective, oral, shorter-course treatment regimens for persons with MDR-TB is much needed and most welcomed.
A Measles Primer
By Andy Pavia, MD and Brian Schwartz, MD
Large outbreaks of measles are occurring in Texas and Ontario, and cases have been diagnosed in several other states and Canadian provinces (see HAN No. 522). Many clinicians have never seen or cared for a patient with measles, so this primer on the recognition and management of measles may be helpful.
Epidemiology
- Before the advent of efficacious vaccines, measles was a leading cause of death among children. In 1963, with a US population roughly 40% of today’s population, measles led to an estimated 48,000 hospitalizations, 1000 cases of encephalitis, and 400-500 deaths.
- Measles is spread by the aerosol route and is one of the most infectious viruses known.
- The measles virus can linger in the air for up to 2 hours after a contagious patient has left a room, such as a waiting room.
- Patients are infectious from the onset of the prodrome until 4 days after the appearance of the rash.
Prevention
- One dose of measles vaccine (available as MMR) is 93% effective at preventing measles. Two doses are 97% effective.
- Measles is very rare in fully vaccinated people.
- Vitamin A has not been shown to prevent measles but may decrease complications in children with measles (see Management).
- Providers should wear N-95 masks around patients, and patients should be placed in negative pressure isolation.
Clinical manifestations
- Measles is a serious illness. It usually follows a predictable course.
- Incubation period: 8-10 days.
- Symptomatic period:
- Prodromal phase
- Fever (often high)
- Cough, non-purulent conjunctivitis, coryza (runny nose) - the “3-Cs”
- Koplik spots (small white papules on the buccal mucosa) may be present
- Rash
- Timing: Begins 3-4 days after the fever
- Distribution: First on the face, usually at the hair line or behind the ears. It spreads to the trunk, then extremities.
- Morphology: Polymorphic, usually a mix of macules and papules. They may feel slightly rough.
- Rashes may be harder to recognize on pigmented skin. See Photos.
- Other clinical manifestations (~20% require hospitalization)
- Otitis media (7-9%)
- Keratitis
- Croup
- Diarrhea
- Pneumonia (1-6%); secondary bacterial pneumonia may occur
- Neurologic complications
- Early encephalitis (often with features of acute disseminated encephalomyelitis); ~1-3/1000
- Measles inclusion body encephalitis: Usually presents within one year of infection.
- Subacute sclerosing panencephalitis: A rare fatal complication presenting 7-10 years after infection.
- Depletion of memory T and B cells. leading to “immune amnesia.”
- Mortality:
- ~1/1000 in otherwise healthy populations
- Children <5 years of age, pregnant patients, and immunocompromised patients are at increased risk of complications and death.
- Prodromal phase
Diagnosis
- Initial management should be based on clinical diagnosis, since serology and PCR testing do not come back in a timely manner.
- Measles IgM Serum: Positive a few days after rash onset. False negatives early on and false positives at low titer can occur.
- Measles PCR of oropharyngeal or nasopharyngeal secretions: Sensitive, usually done via public health. Urine PCR may be positive.
Management
- Supportive care: Hydration, pain control, corneal lubrication for eye irritation, respiratory support, and remaining alert to possible secondary bacterial infection.
- Prophylactic antibiotics: Not indicated.
- Vitamin A: Shown to reduce mortality in malnourished children, but the benefits for well-nourished children are unknown. Notwithstanding, AAP recommends vitamin A once daily for 2 days for all children with measles.
- ≥12 months: 200,000 IU (60,000 µg retinol activity equivalents [RAE])
- 6-11 months: 100,000 IU (30,000 µg RAE)
- <6 months: 50,000 IU (15,000 µg RAE)
- Antivirals: Not indicated, none with proven benefit.
- There is no data supporting any alternative therapies (i.e. cod liver oil, steroids).
For Further Information
Photos
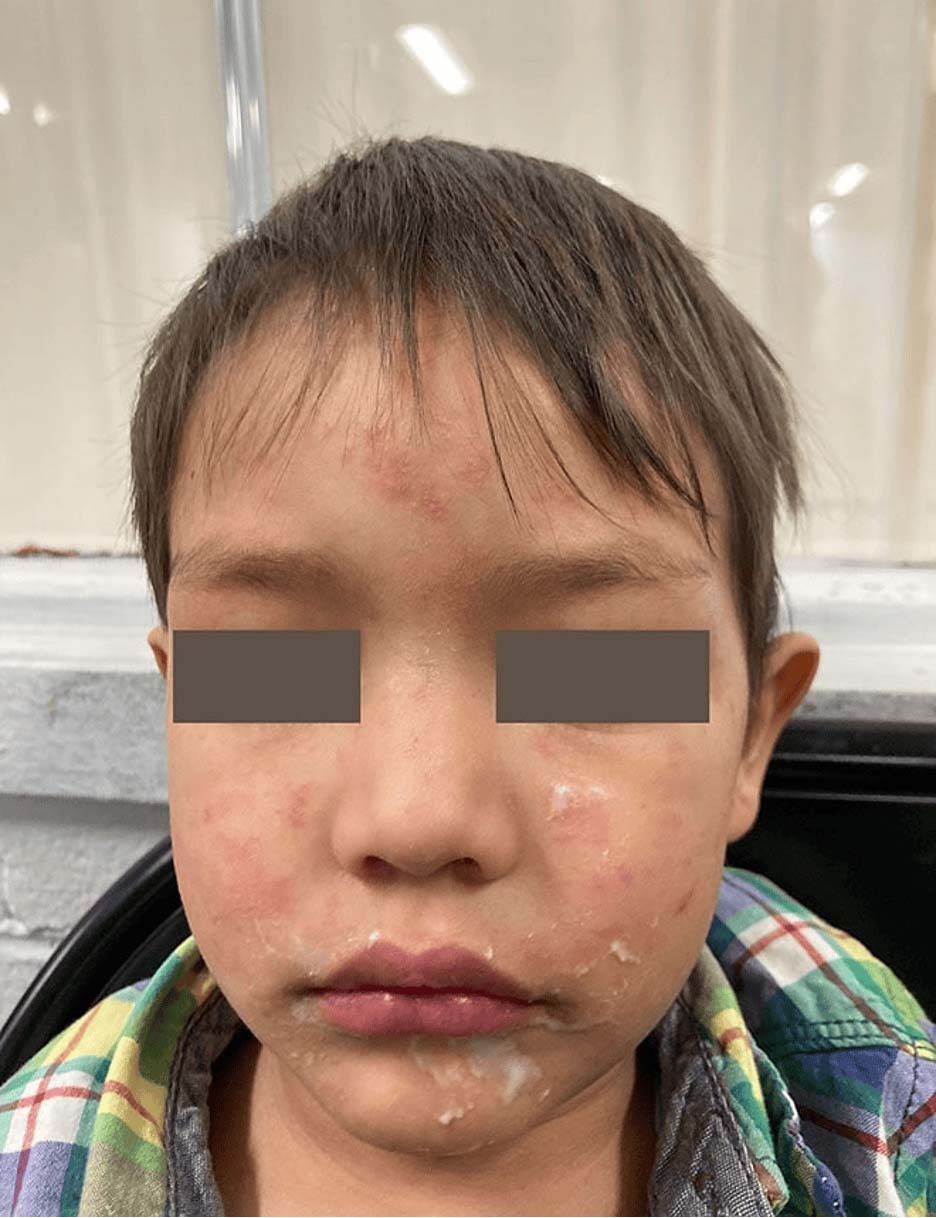
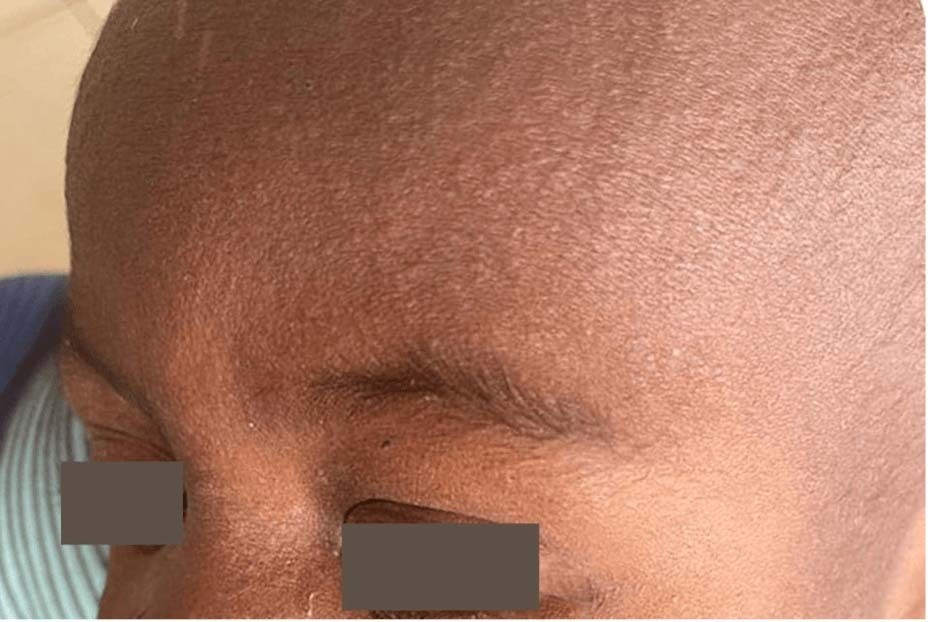
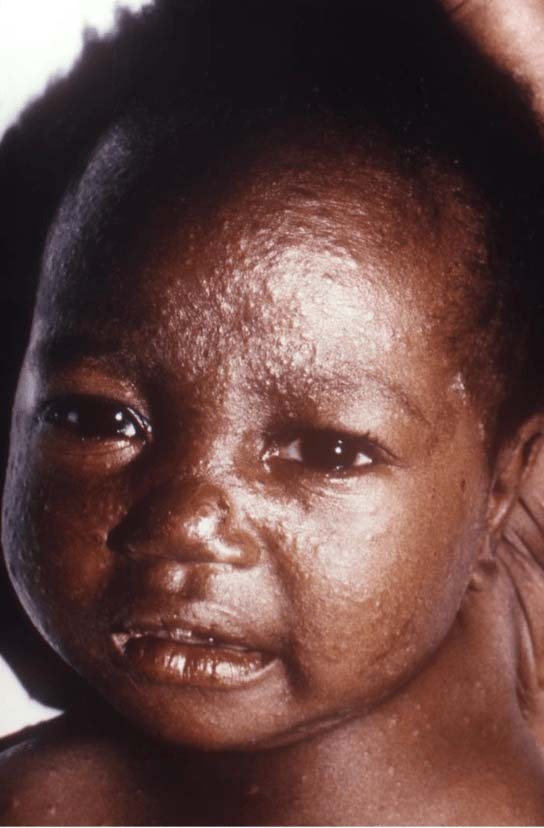
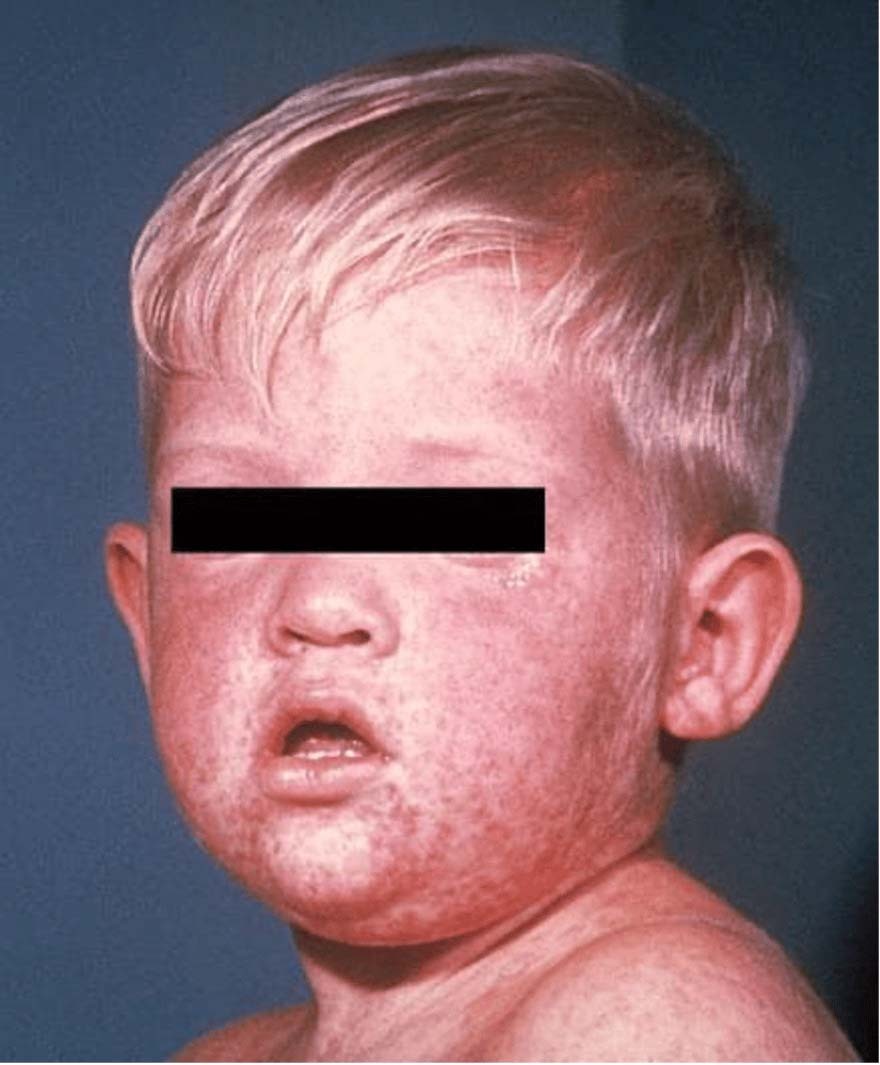
Photo Source (all): CDC
AMS Pearl: Handshake Stewardship for Surgery Patients
- A single center study conducted at a large academic medical center evaluated the use of handshake stewardship in adult surgery patients.
- An infectious diseases physician and pharmacist reviewed antimicrobials prescribed to surgical patients once weekly and provided in person recommendations to the physician associates and nurse practitioners providing care for the patients.
- Discontinuation, de-escalation, and escalation of therapy were the most frequent recommendations, and the acceptance rate was 72%. Overall antimicrobial days of therapy/1,000 days present significantly decreased while there was no difference in ICU length of stay, 30-day readmission, and hospital-onset Clostridioides difficile infection. Hospital length of stay was increased, but it did not appear to be a result of the intervention.
- Handshake stewardship strategies can be labor intensive, but this study demonstrates that a once-weekly effort can be beneficial. AMS programs could consider the strategy used in this study to start a similar program. Antimicrob Steward Healthc Epidemiol. 2025 Feb 12;5(1):e46. doi: 10.1017/ash.2024.498.
Antimicrobial Shortages (US)
- Recent shortages:
- Rifapentine 150 mg tablets (25 Feb 2025) NEW
- Resolved shortages:
- Ampicillin injection (24 Feb 2025) NEW
- Ofloxacin 0.3% ophthalmic solution (3 Feb 2025)
- Antimicrobial drugs or vaccines in continued reduced supply or unavailable (as of 8 March 2025) due to increased demand, manufacturing delays, product discontinuation by a specific manufacturer, or unspecified reasons:
- Antibacterial drugs:
- Aminoglycosides:
- Gentamicin injection (22 Feb 2021)
- Azithromycin oral suspension, 1 gm packets (20 Nov 2024)
- Bacitracin ophthalmic ointment 500 units/gm (12 Sep 2024)
- Cephalosporins:
- Cefazolin injection (4 Jun 2018)
- Cefdinir 300 mg capsules (29 Jun 2023)
- Cefdinir 125 mg/5 mL, 250 mg/5 mL oral suspension (29 Jun 2023)
- Cefotaxime injection (10 Jun 2015)
- FDA is allowing temporary importation of product from SteriMax in Canada, in conjunction with Provepharm Life Solutions and its distributor Direct Success. Click here for details.
- Chloramphenicol injection (9 Oct 2023)
- Clindamycin phosphate injection (25 Jun 2015)
- Fluoroquinolones:
- Ciprofloxacin injection (13 Jan 2023)
- Levofloxacin injection in D5W (29 May 2024)
- Levofloxacin oral solution, 25 mg/mL (15 Sep 2023)
- Moxifloxacin 400 mg tablets (6 Dec 2023)
- Glycopeptides, glycolipopeptides, lipopeptides:
- Vancomycin injection (1 Jun 2015)
- Metronidazole injection (20 Oct 2021)
- Neomycin and Polymyxin B Sulfates GU Irrigant (25 Jun 2023)
- Nitrofurantoin oral suspension (5 Jun 2018)
- Oxazolidinones:
- Linezolid injection (16 Oct 2024)
- Penicillins:
- Amoxicillin, all oral formulations (18 Oct 2022)
- Amoxicillin-clavulanate, all oral formulations (17 Nov 2022)
- Dicloxacillin 250 mg, 500 mg capsules (18 Aug 2021)
- Nafcillin injection (20 Mar 2024)
- Penicillin G benzathine injection (1 Feb 2023) Availability update here
- Penicillin G benzathine/Penicillin G procaine (31 Mar 2023) Availability update here
- Penicillin VK oral solution 250 mg/5 mL (17 May 2023)
- Penicillin VK 250 mg, 500 mg tablets (17 May 2023)
- Rifaximin 200 mg tablets (11 Apr 2024)
- Aminoglycosides:
- Antifungal drugs:
- Amphotericin B Lipid Complex (5 Aug 2022)
- Fluconazole injection (9 Aug 2024)
- Ibrexafungerp 150 mg tablets (3 Dec 2024)
- Nystatin oral suspension (21 June 2024)
- Antimycobacterial drugs:
- Isoniazid 100 mg, 300 mg tablets (1 Sep 2022)
- Antiparasitic drugs:
- Mefloquine 250 mg tablets (14 May 2024)
- Nitazoxanide oral susp 100 mg/5 mL (15 Feb 2024)
- Antiviral drugs:
- Cidofovir injection (01 Nov 2024)
- Oseltamivir 30 mg, 45 mg, 75 mg capsules (1 Nov 2022)
- Oseltamivir powder for oral suspension (1 Nov 2022)
- Peginterferon alfa-2a (Pegasys) (8 Jan 2025)
- Ribavirin for inhalation solution (23 May 2023)
- Antibacterial drugs:
- Antimicrobial drugs recently discontinued:
- Bezlotoxumab injection (31 Jan 2025, by Merck)
- Posaconazole oral susp 40 mg/mL (Dec 2023, by Merck)
- Sulfacetamide 10%/Prednisolone acetate 0.2% oph ointment (Aug 2023 by Allergan, sole supplier)
- Penicillin G procaine 600,000 units/mL IM injection (Jun 2023)
- Ritonavir oral solution 80 mg/mL (Jan 2023)
- For more detailed information including estimated resupply dates, see https://www.ashp.org/Drug-Shortages/

